Environmental and Health Concerns: Characteristics of nanoparticles that are relevant for health effects
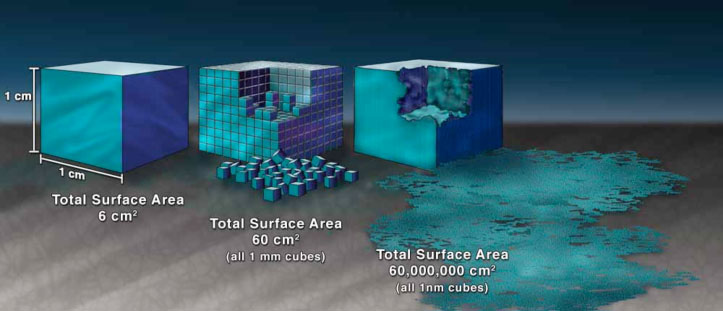
Illustration demonstrating the effect of the increased surface area provided by nanostructured materials.
Exposures to nanomaterials can involve a wide range of nanomaterial sizes, shapes,functionalities, concentrations, chemical compositions, and exposure frequencies or durations.
- Size – Due to their size, nanoparticles are able to cross cell membranes, reach the blood and various organs. Due to their small size engineered nanomaterials may gain access to regions of the body that are normally protected from xenobiotics (sanctuaries), such as the brain.
- Shape – health effects of nanoparticles can also depend on their shape. One example is carbon nanotubes that shows abestos like effects.
- Chemical composition and surface characteristics – Hazard identification has revealed that the physico-chemical properties of engineered nanomaterials can greatly influence their uptake. Engineered nanomaterials show greater uptake and are more biologically active than larger-sized particles of the same chemistry, due to their greater surface area per mass possibly making them more toxic than larger particles of the same composition. Surface modifications can prolong engineered nanomaterials circulation in blood, enhance uptake at a target site, affect translocation, and alter excretion.
- Dose -Exposure in experimental studies is typically expressed as dose, usually on a mass/subject body weight basis, or as concentration. The relatively small amount of literature has generally shown dose- or concentration-response relationships, as is usually the case for toxicity endpoints.
- Persistence- The persistence of engineered nanomaterials may be a major factor contributing to their effects. Many nanomaterials are designed to be mechanically strong and resist degradation
Toolbox
- Image: https://www.nano.gov/nanotech-101/special
- https://www.cdc.gov/niosh/docs/2012-147/pdfs/2012-147.pdf
- Yokel, R. A., & MacPhail, R. C. (2011). Engineered nanomaterials: exposures, hazards, and risk prevention. Journal of Occupational Medicine and Toxicology (London, England), 6, 7. http://doi.org/10.1186/1745-6673-6-7